For formulators in the food, cosmetic, and pharmaceutical industries, confusing Span 80 and Tween 80 is a common initial hurdle. They share a similar lineage and are often sold side-by-side, yet they behave like polar opposites in a mixture.
Both are non-ionic surfactants derived from sorbitan (a natural compound from corn or wheat), but their chemical modifications determine whether they “love” water or oil. Understanding this distinction is critical for creating stable emulsions, whether you are formulating a fluffy mousse or a heavy industrial lubricant.
1. Physical Appearance and Solubility
Before diving into the chemistry, you can often distinguish these two by how they look and how they interact with solvents.

- Span 80 (Sorbitan Monooleate):
- Appearance: A light yellow to amber, viscous, oily liquid.
- Solubility: It is lipophilic (oil-loving). It generally disperses in water but does not dissolve; however, it dissolves easily in most organic solvents and oils.
- Tween 80 (Polysorbate 80):
- Appearance: A lemon-to-amber colored, viscous liquid.
- Solubility: It is hydrophilic (water-loving). It is soluble in water and ethanol but is generally insoluble in vegetable oils and mineral oils.
2. Chemical Structure: The Role of Ethoxylation
The fundamental difference lies in a chemical process called ethoxylation.
Span 80: The “Anchor”
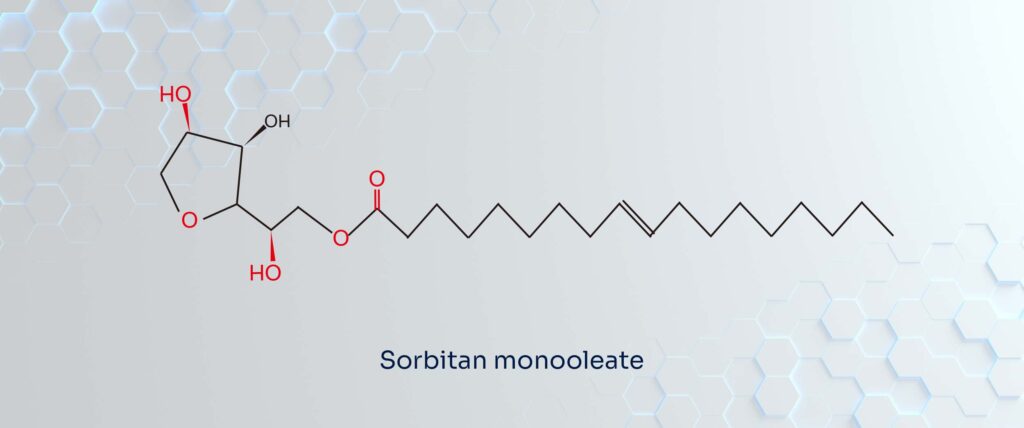
Span 80 is chemically known as Sorbitan Monooleate. It is a simpler molecule formed by esterifying sorbitol with oleic acid. Structurally, it lacks polyoxyethylene chains. Because of the long hydrocarbon chain from the oleic acid, the molecule is dominated by non-polar characteristics, making it hydrophobic (water-repelling).
Tween 80: The “Tentacles”
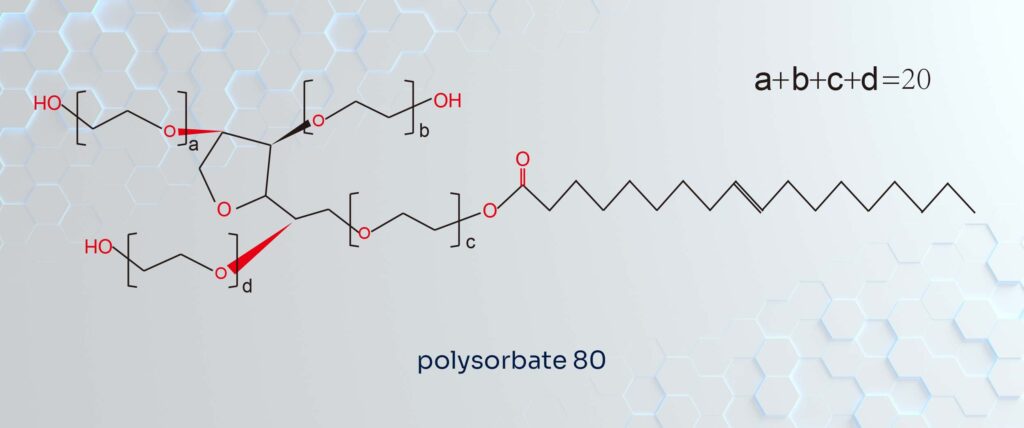
Tween 80, or Polysorbate 80, starts as Span 80 but undergoes a crucial transformation. It is produced by reacting the sorbitan ester with ethylene oxide. This reaction grafts polyoxyethylene chains onto the molecule.
To visualize this, imagine the relationship like a magnet:
- Span 80 acts like the end of a magnet that bonds tightly with oil. It swims comfortably in lipids.
- Tween 80 acts like the end that reaches out to water. Those added polyoxyethylene chains function like long “tentacles” that grab onto water molecules.
The Mechanism: Why Structure Dictates Function
To truly understand how these surfactants work, we must look at the specific chemical groups that act as “anchors” and “buoys” within the mixture. It is a tug-of-war between the tail and the head of the molecule.
- The Lipophilic Tail (The Anchor):Both molecules share a long fatty acid hydrocarbon chain (derived from oleic acid). This non-polar “tail” is chemically similar to fats and oils. Because “like dissolves like,” this tail naturally buries itself inside the oily phase. It acts as an anchor, holding the surfactant molecule firmly within the oil droplets.
- The Hydrophilic Head (The Buoy):The difference lies in how the “head” of the molecule interacts with water.
- In Span 80, the water attraction comes only from the few free hydroxyl (-OH) groups on the sorbitan ring. These are weak compared to the massive fatty tail, so the molecule remains oil-soluble.
- In Tween 80, the ethoxylation process adds long chains rich in oxygen-carbon (C-O-C) ether linkages and terminal hydroxyl (-OH) groups. These groups are strongly polar. They readily form hydrogen bonds with water molecules, creating a powerful “pull” toward the aqueous phase. This pull is strong enough to overpower the drag of the fatty tail, making Tween 80 water-soluble.
3. The HLB Value: The Scientific Differentiator
The industry standard for distinguishing these surfactants is the Hydrophilic-Lipophilic Balance (HLB) system, which measures the degree to which a surfactant is hydrophilic or lipophilic.
| Surfactant | HLB Value | Nature | Best For |
|---|---|---|---|
| Span 80 | ~4.3 | Highly Lipophilic | Water-in-Oil (W/O) emulsions. It helps disperse water droplets into a continuous oil phase. |
| Tween 80 | ~15.0 | Highly Hydrophilic | Oil-in-Water (O/W) emulsions. It helps disperse oil droplets into a continuous water phase. |
4. Comparison of Applications
Because of their HLB values, they are used in opposing (but often complementary) ways across industries.

Food Industry
- Tween 80: Used to stabilize ice creams (preventing ice crystals) and homogenize sauces or dressings where oil must stay suspended in water.
- Span 80: Used in margarine, shortenings, and bakery products where water needs to be dispersed within a solid fat or oil matrix.
Cosmetics & Personal Care
- Tween 80: Found in lightweight lotions, shampoos, and body washes to blend fragrance oils into watery bases.
- Span 80: Preferred for “heavy” formulations, such as rich night creams, ointments, and diaper creams that rely on a fatty base to protect the skin.
Pharmaceuticals
- Tween 80: Critical for solubilizing drugs in liquid vaccines, injectables, and oral suspensions.
- Span 80: Used as an excipient in rectal suppositories and topical ointments where a slow-release, oil-based delivery is required.
Industrial Insight: Corrosion Inhibition
An interesting industrial application involves using both in bioethanol-gasoline blends. Research suggests that a mixture of Span 80 and Tween 80 can tightly adhere to metal surfaces. This creates a hydrophobic barrier that prevents water from reaching the metal, significantly reducing corrosion in fuel storage tanks.
5. The Power of Blending: Synergy
While they are different, Span 80 and Tween 80 are often the “Power Couple” of formulation.
Using a single surfactant can sometimes result in an unstable emulsion that separates over time. By blending a hydrophilic surfactant (Tween) with a hydrophobic one (Span), formulators can create a more tightly packed interface between the oil and water droplets.
Common Strategy:
- To stabilize a complex cream, a formulator might use a specific ratio of Span 80 and Tween 80 to hit a “Target HLB” (e.g., HLB 9 or 10) that perfectly matches the oil phase of their product. This “hand-holding” effect ensures the oil and water remain locked together for a longer shelf life.
Conclusion: How to Choose?
When selecting between the two, consult this simple decision matrix:
- Identify the Primary Phase: Is your product mostly water (use Tween 80) or mostly oil (use Span 80)?
- Desired Texture: Do you want a pourable milk (Tween) or a thick paste (Span)?
- Regulatory: Ensure compliance (FDA/EFSA) for your specific application region.
Summary: Think of Tween 80 as the “Water-Lover” and Span 80 as the “Oil-Lover.” When used correctly, they are the hands that hold your formulation together.

